Provide Your Patients With COVID-19 Testing
FDA Compliant/CLIA certified lab/ Covid test with EUA
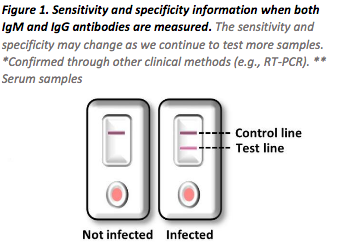
Not just a Rapid Test …a fully compliant testing program
Fast & Accurate
- One drop of blood, results in ten minutes
- Tests for both IgG and IgM antibodies
Accredited
- EUA Authorized May 29
Insured
- CLIA high complexity lab umbrella coverage at no charge
Cost Effective
- PCR swabs for IgM positive patients provided at no charge • Your test results reported to CDC weekly by CLIA lab
Sensitivity: IgG 96.7%; IgM 100%; Combined 100%
Specificity: IgG 97.5%; IgM 100%; Combined 97.5%
Through our lab partnership, we provide the only fully compliant program for you to be able to test staff, patients and your community for Covid-19 IgG and IgM antibodies. We’ve partnered with a national high complexity CLIA lab to ensure all parties are compliant with FDA testing regulations. Once you have placed your order, you will receive our CLIA laboratory partner on-board form to complete so that you’ll be covered under a high complexity CLIA umbrella.
Q&A
Yes, they are combined together into a single cassette. There is a separate window to
place the blood sample for IgG and IgM that are clearly marked.
Since the patient may not know when they are infected, it is important to test for both IgM
and IgG.
This is not an over-the-counter product for home use. The test must be administered by a licensed medical professional. However, it is a POC (point of care) test that does not
require sending samples to a lab. Therefore, the results can be obtained on-site at a
clinic.
There is no known LOD of this assay. The kit is qualitative and is based on antigenantibody
interactions, and every person has different antibody affinities.
The test cartridges and diluent provided in the finger prick and serum/whole blood kits
are identical. The only difference is that the finger prick kit comes with materials
necessary for capillary blood collection (lancets, alcohol swabs, bandages).
The billing code for non-CDC laboratory tests for SARS-CoV-2/COVID-19 is 86328.
The patient’s insurance company should be contacted to determine coverage and pricing.
These kits are for in vitro diagnostic use and have been submitted for Emergency Use
Authorization following guidance from the FDA on March 16, 2020. The tests have not
been reviewed by the FDA and results from antibody testing should not be used as the
sole basis to diagnose or exclude SARS-CoV-2 (COVID-19) infection or to inform
infection status.
Our IgG/IgM rapid test kit have a 84.1% sensitivity (true positive) and a 92.3% specificity
(true negative) rate.
We have ready inventory and can ship today.
$25 per kit x 25 kits ($625 per case) One case minimum order
To order product, please fill out the form using the link below.



